A) CaCO3
B) CO2
C) CH4
D) NaHCO3
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 2.35-mole sample of H2O2 weighs
A) 79.9 g
B) 2.35 g
C) 36.4 g
D) 42.3 g
E) 42.3 amu
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which represents the greatest mass?
A) 1.0 mol Rb
B) 1.0 mol Ti
C) 1.0 mol Fe
D) 1.0 mol Al
E) all the same
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Convert: 4.13 mol PCl5 = ___________ molecules PCl5
A) 2.49 *1024
B) 6.86 * 10-24
C) 1.46 *1023
D) 1.19 * 1022
E) none of these
G) B) and D)
Correct Answer

verified
Correct Answer
verified
True/False
The mole can be defined as the number equal to the number of oxygen atoms in 32.00 g of oxygen.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Vinegar contains carbon, hydrogen, and oxygen with percent masses of 40.01% C and 6.70% H. If the molar mass of vinegar is about 60 g/mol, what is its molecular formula?
A) CH2O
B) C3H7O3
C) C3H8O
D) C2H20O
E) C2H4O2
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the percent (by mass) of carbon in glucose, C6H12O6.
A) 40.0 %
B) 53.3 %
C) 25.0 %
D) 6.7 %
E) none of these
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many grams of fluorine are contained in 5 molecules of boron trifluoride?
A) ![]() g
g
B) ![]() g
g
C) ![]() g
g
D) ![]() g
g
E) 6.022 * 1023 g
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mass percent of hydrogen in NH4Br.
A) 4.12 %
B) 1.03 %
C) 5.0 %
D) 16.5 %
E) none of these
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molar mass of ammonium phosphate is
A) 149.09 g/mol
B) 113.01 g/mol
C) 302.95 g/mol
D) 165.09 g/mol
E) 133.09 g/mol
G) B) and E)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Consider equal mole samples of dinitrogen monoxide, aluminum nitrate, and potassium cyanide. Rank these from least to greatest number of nitrogen atoms in each sample.
A) potassium cyanide, aluminum nitrate, dinitrogen monoxide
B) aluminum nitrate, dinitrogen monoxide, potassium cyanide
C) potassium cyanide, dinitrogen monoxide, aluminum nitrate
D) aluminum nitrate, potassium cyanide, dinitrogen monoxide
E) dinitrogen monoxide, aluminum nitrate, potassium cyanide
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The empirical formula for acetic acid is CH2O. Its molar mass is 60 g/mol. The molecular formula is
A) CH2O
B) C2H4O2
C) C2H6O
D) C2HO2
E) none of the above
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of phosphorus are present in 80.0 g of phosphorus?
A) 0.387 mol
B) 2.58 mol
C) ![]() mol
mol
D) ![]() mol
mol
E) none of these
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 1.50-mol sample of Zr represents how many atoms?
A) 9.03 * 1023 atoms
B) 2.49 *10-24 atoms
C) 4.01 *1023 atoms
D) 8.24 * 1025 atoms
E) 1.64 *10-2 atoms
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Convert: 0.205 mol Ca(OH) 2 = ___________ g Ca(OH) 2
A) 15.2
B) 2.77 *10-3
C) 11.7
D) 361
E) none of these
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the number of moles of Cl2 molecules in a sample that contains 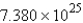 molecules of Cl2.
molecules of Cl2.
A) ![]() mol
mol
B) ![]() mol
mol
C) ![]() mol
mol
D) 122.6 mol
E) none of these
G) C) and D)
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Calculate the number of moles in 2.28 g Rb2C2O4.
A) ![]() mol
mol
B) 113.6 mol
C) ![]() mol
mol
D) ![]() mol
mol
E) ![]() mol
mol
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A compound contains 40.0% carbon, 6.7% hydrogen, and 53.3% oxygen (by mass) . Calculate the empirical formula.
A) CH2O
B) C2H2O
C) CH4O
D) C3H6O3
E) C2HO2
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molar mass of C3H6O3?
A) 90.09 g/mol
B) 94.08 g/mol
C) 54.06 g/mol
D) 84.03 g/mol
E) none of these
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many atoms of phosphorus are present in 40.8 g of phosphorus?
A) ![]() atoms
atoms
B) ![]() atoms
atoms
C) ![]() atoms
atoms
D) ![]() atoms
atoms
E) 1.32 atoms
G) A) and C)
Correct Answer

verified
A
Correct Answer
verified
Showing 1 - 20 of 120
Related Exams