A) the fact that electrons can move very quickly from one resonance form to another.
B) adjacent 2pz orbitals each having one electron.
C) Wade's rules.
D) double-headed arrows.
E) Cooper pairs.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Draw a few subunits of the polymer derived 3-aminopropanoic acid,which is also known as -alanine. 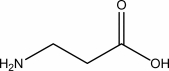
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many structural isomers does xylene,C6H4(CH3) 2,have?
A) None-i.e.,there is only 1 molecule
B) 2
C) 3
D) 4
E) 5
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is NOT true about n-alkanes?
A) Their formulas are all CnH2n+2.
B) All the carbon atoms are centers of tetrahedral geometry.
C) Their melting points increase with the number of carbon atoms.
D) Their vapor pressures increase with the number of carbon atoms.
E) All of the carbons are sp3 hybridized.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following do you predict has the highest fuel value?
A) benzene,C6H6
B) cyclohexane,C6H12
C) 1-hexene,C6H12
D) 1-hexyne,C6H10
E) hexane,C6H14
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is not a structural isomer of octane?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecular structures is probably the most stable?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A homopolymer is a polymer in which ________
A) each polymer has the same mass.
B) each polymer has the same mass and structure.
C) the monomers are distributed uniformly throughout the polymer.
D) the polymers are distributed uniformly throughout the solution.
E) there is only one type of monomer unit.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the degree of unsaturation of CH2  CH - C
CH - C  CH?
CH?
A) 1
B) 2
C) 3
D) 4
E) 5
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements regarding alkenes and alkynes is NOT correct?
A) Alkenes and alkynes are typically present in large amounts in crude oil.
B) Alkenes are commonly derived from plant sources.
C) Alkynes are not common in nature due to the reactivity of the C ![]() C bond.
C bond.
D) Alkanes can be dehydrogenated to give alkenes and alkynes.
E) Hydrocarbon reactivity increases in the order alkanes < alkenes < alkynes.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the systematic name of the molecule shown? 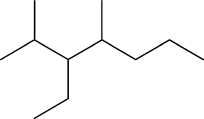
A) 4-methyl-5-isopropylheptane
B) 3-propyl-4-methylheptane
C) 2-methyl-3,4-diethylpentane
D) 2,4-dimethyl-3-ethylheptane
E) 3-ethyl-2,4-dimethylheptane
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which functional group is found in the following molecule? 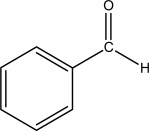
A) aldehyde
B) carboxylic acid
C) ester
D) ether
E) ketone
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What functional group is found in the following molecule? 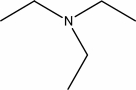
A) amine
B) amide
C) ammonia
D) ammonium
E) amonyl
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Do carbon atoms in aromatic hydrocarbon molecules (not ions) obey the octet rule?
A) No,each carbon is bonded to only three atoms,and there are no lone pairs.
B) No,each carbon has one single bond and one double bond for a total of only six electrons.
C) Yes,each carbon obeys the octet rule.
D) Yes,but only because the double bond is counted twice owing to the resonance structures.
E) Yes,but only when one or the other resonance form dominates,not when the structure is an average of the two.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many structural (constitutional) isomers are there for propylene,and why?
A) two,because of resonance
B) two,because of geometrical isomerism
C) two,because the double bond can be in either one of two locations
D) none,because the two skeletal structures can be superimposed
E) none,because double bonds always occur on the right in alkenes
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the by-product of the preparation of nylon?
A) H+
B) CO2
C) hydrogen gas
D) water
E) ammonia
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Consider the isomers of C5H12.Draw the skeleton structure for the isomer that will have the highest boiling point.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular formula for the following compound? 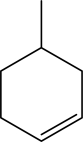
A) C6H11
B) C6H12
C) C7H11
D) C7H12
E) C7H14
G) None of the above
Correct Answer

verified
Correct Answer
verified
Showing 161 - 178 of 178
Related Exams