A) n = 2, l = 1, ml = +1
B) n = 4, l = 2, ml =-1
C) n = 3, l =3, ml = -1
D) n = 1, l = 0, ml =0
E) n = 3, l = 2, ml =0
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the wavelength (in nm) of the red light emitted by a neon sign with a frequency of 4.76 × 1014 Hz.
A) 630 nm
B) 159 nm
C) 476 nm
D) 776 nm
E) 176 nm
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What value of l is represented by a f orbital?
A) 1
B) 2
C) 0
D) 3
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the end (final) value of n in a hydrogen atom transition,if the electron starts in 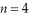 and the atom emits a photon of light with a wavelength of 486 nm.
and the atom emits a photon of light with a wavelength of 486 nm.
A) 1
B) 5
C) 3
D) 4
E) 2
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the numbers for ml for an f orbital.
A) 0, +1, +2, +3, +4
B) +1, +2, +3
C) -3, -2, -1, 0, +1, +2, +3
D) -2, -1, 0, +1, +2
E) -2, -1, 0
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Define constructive interference.
Correct Answer

verified
Waves comb...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
The de Broglie wavelength of an electron with a velocity of 6.90 × 106 m/s is ________ m.The mass of the electron is 9.11 × 10-28 g.
A) 9.49 × 109
B) 9.49 × 1012
C) 1.05 × 10-16
D) 1.05 × 10-13
E) 1.05 × 10-10
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the wavelength of a baseball (m = 155 g) moving at 32.5 m/s.
A) 7.60 × 10-36 m
B) 1.32 × 10-34 m
C) 2.15 × 10-32 m
D) 2.68 × 10-34 m
E) 3.57 × 10-32 m
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following visible colors of light has the highest frequency?
A) green
B) red
C) blue
D) yellow
E) orange
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many orbitals are there in the seventh shell?
A) 2
B) 6
C) 21
D) 49
E) 7
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the energy of the violet light emitted by a hydrogen atom with a wavelength of 410.1 nm.
A) 4.85 × 10-19 J
B) 2.06 × 10-19 J
C) 1.23 × 10-19 J
D) 8.13 × 10-19 J
E) 5.27 x 10-19 J
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Describe the shape of a 2px orbital.
A) spherical
B) dumbbell shaped
C) three balls
D) four balls
E) eight balls
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the possible values of n and ml for an electron in a 4d orbital?
A) n = 1, 2, 3, or 4 and ml = 2
B) n = 1, 2, 3, or 4 and ml = -2, -1, 0, +1, or +2
C) n = 4 and ml = 2
D) n = 5 and ml = -2, -1, 0, +1, +2
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the energy change associated with the transition from 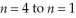 in the hydrogen atom.
in the hydrogen atom.
A) +4.89 × 10-18 J
B) +1.64 × 10-18 J
C) -6.12 × 10-18 J
D) +3.55 × 10-18 J
E) -2.04 × 10-18 J
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Showing 121 - 134 of 134
Related Exams