Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following structures represents a different compound from the other three?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the 1,3-diaxial strain for an ethyl group is 4.0 kJ/mol,what is the energy difference between the axial and equatorial conformations of ethylcyclohexane?
A) 2.0 kJ/mol
B) 4.0 kJ/mol
C) 8.0 kJ/mol
D) 16.0 kJ/mol
E) Cannot be determined from the 1,3-diaxial strain
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following cycloalkanes has the most ring strain?
A) cyclopropane
B) cyclobutane
C) cyclopentane
D) cyclohexane
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds can adopt a chair conformation in which there are no axial methyl groups?
A) 1,1-dimethylcyclohexane
B) cis-1,2-dimethylcyclohexane
C) trans-1,2-dimethylcyclohexane
D) trans-1,3-dimethylcyclohexane
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true regarding the conformation of substituted cyclohexanes?
A) ring flip of substituted cyclohexanes flips the axial and equatorial positions of substituents
B) substituted cyclohexanes are destabilized by 1,3-diaxial interactions
C) the twist-boat conformation of cyclohexane is more stable than the chair conformation
D) the relative amount of two conformations of substituted cyclohexanes can be determined from the difference in strain energy
F) B) and C)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
In which of the following compounds would the carbon-carbon bond angle diverge the greatest from 109 ?
A) cyclodecane
B) cyclooctane
C) cyclopentane
D) cyclopropane
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In cycloheptane,which of the following factors contributes the least to the stability of the ring conformation?
A) torsional strain
B) angle strain
C) steric strain
D) all of these contribute to an approximately equal degree
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following structures represents trans-1,3-dimethylcyclohexane?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following structures represents trans-1,2-dimethylcyclohexane?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Substitution of which of the following groups on a cycloalkane would result in the greatest amount of steric strain?
A) bromo
B) ethyl
C) isopropyl
D) hydroxyl
F) All of the above
Correct Answer

verified
Correct Answer
verified
Essay
Instructions: (-)-Menthol is responsible for the characteristic flavor and taste of peppermint.The structure of (-)-menthol is shown below.Use this information to answer the following question.
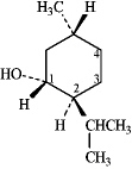 -Refer to instructions.On the chair template provided below,draw the two chair conformations that are in equilibrium for (-)-menthol.
-Refer to instructions.On the chair template provided below,draw the two chair conformations that are in equilibrium for (-)-menthol. 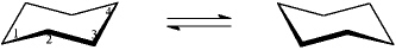
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following cycloalkanes would be the most similar to its open chain counterpart?
A) cyclodecane
B) cyclooctane
C) cyclopentane
D) cyclopropane
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following structures represents a different compound from the other three?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the most stable conformation of cis-1-isopropyl-3-methylcyclohexane?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) None of the above
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
If cyclohexane were planar,how much torsional strain would be present in the planar molecule? Assume that the energy cost for each H«H eclipsing interaction is 4.0 kcal/mol.
A) 12.0 kJ/mol
B) 24.0 kJ/mol
C) 36.0 kJ/mol
D) 48.0 kJ/mol
E) 64.0 kJ/mol
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the most stable conformation of trans-1-ethyl-3-methylcyclohexane?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) A) and C)
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
What is the energy difference between the two chair conformations of the following compound that is due to steric strain?
Cis-1-bromo-4-isopropylcyclohexane

A) 7.2 kJ/mol
B) 5.6 kJ/mol
C) 9.2 kJ/mol
D) 11.2 kJ/mol
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 18 of 18
Related Exams