A) acidic because NaC2H3O2 is a strong acid
B) basic because NaC2H3O2 is a weak base
C) neutral because there is no hydrolysis
D) basic because NaC2H3O2 is the salt of a weak acid
E) acidic because NaC2H3O2 is the salt of a weak base
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following salts,when dissolved in water,produces the solution with the highest pH?
A) RbI
B) RbBr
C) RbCl
D) RbF
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At 25 °C,the pH of pure water is:
A) 0
B) >0,<7
C) 7
D) >7,<14
E) 14
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which species in the following reaction acts as a Lewis acid? CuSO4(s) + 4 NH3(aq) ⇌ [Cu(NH3) 4]2+(aq) + SO42-(aq)
A) SO42-
B) Cu2+
C) [Cu(NH3) 4]2+(aq)
D) NH3
E) [Cu(NH3) 4]2+(aq) and SO42-
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH of a 0.375 M aqueous solution of benzoic acid? Ka = 6.3 × 10-5
A) 8.9
B) 5.1
C) 2.3
D) 0.43
E) 11.7
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
pOH = 3.14 is equivalent to:
A) pH = 11
B) [H+] = 1.4 × 10-10 M
C) [OH-] = 7.2 × 10-4 M
D) [H+} = 7.0 × 10-4 M
E) [OH-] = 3.14 × 10-7 M
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the pH of 263 ml of an aqueous solution which has [NH4I] = 0.300 M.Kb = 1.74 × 10-5 for NH3(aq) .
A) 2.6
B) 11.4
C) 4.9
D) 9.1
E) 4.6
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the Br∅nsted-Lowry acids and bases in the following equation: H2O + NH2- ⇌ NH3 + OH-
A) acids H2O,OH- bases NH3,NH2-
B) acids NH2-,NH3 bases H2O,OH-
C) acids H2O,NH2- bases OH-,NH3
D) acids NH3,NH2- bases OH-,H2O
E) acids H2O,NH3 bases NH2-,OH-
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the [Cl-] of a solution prepared by dissolving 0.1824 g of hydrogen chloride in sufficient pure water to prepare 500.0 ml of solution?
A) 1.00 × 10-2 M
B) 1.00 × 10-8 M
C) 1.00 × 10-12 M
D) 1.00 × 10-4 M
E) 1.0 M
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a logical inference from the fact that a 0.10 M solution of potassium acetate,KC2H3O2,is less alkaline than a 0.10 M solution of potassium cyanide,KCN?
A) Hydrocyanic acid is a weaker acid than acetic acid.
B) Cyanides are less soluble than acetates.
C) Hydrocyanic acid is less soluble in water than acetic acid.
D) Acetic acid is a weaker acid than hydrocyanic acid.
E) 0.10 M potassium acetate is more concentrated than 0.10 M potassium cyanide.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the pH of a 1.60 mol L-1 CH3NH3Cl solution.Kb for methylamine,CH3NH2,is 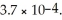
A) 1.61
B) 5.18
C) 8.82
D) 12.39
F) B) and C)
Correct Answer

verified
Correct Answer
verified
True/False
H2SO4 is a weaker acid than H2SO3.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
The term pH = -ln [H+].
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When dissolved in water,which compound is generally considered to be an Arrhenius acid?
A) CH3CO2H
B) NaOH
C) Na2CO3
D) CH3CH2OH
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For HI,predict whether the solution is acidic,basic or neutral and why.
A) acidic because HI is a strong acid
B) basic because HI is a weak base
C) neutral because there is no hydrolysis
D) basic because HI is the salt of a weak acid
E) acidic because HI is the salt of a weak base
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution has pOH of -0.47.This means that:
A) the solution has a pH of 13.53
B) the solution has an [OH-] = 0.34 M
C) the solution has an [OH-] greater than 10.0 M
D) the solution has an [OH-] = 2.95 M
E) The solution has an [H+] = 2.95 M
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which indication of relative acid strengths is INCORRECT?
A) HCl > HF
B) HClO2 > HClO
C) H2SO4 > H2SO3
D) H2SO3 > HNO3
E) CH3CO2H > CH3CH2OH
G) A) and E)
Correct Answer

verified
Correct Answer
verified
True/False
According to the Arrhenius theory,a neutralization reaction involves the combination of an acid with a base to make only water.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The acid-dissociation constant of hydrocyanic acid (HCN) at 25.0 °C is 4.9 × 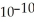 .What is the pH of an aqueous solution of 0.080 mol L-1 sodium cyanide (NaCN) ?
.What is the pH of an aqueous solution of 0.080 mol L-1 sodium cyanide (NaCN) ?
A) 11.11
B) 2.89
C) 1.3 × 10-3
D) 7.8 × 10-12
E) 3.9 × 10-11
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the pH of a 0.800 mol L-1 aqueous NaCH3CO2 solution.Ka for acetic acid,CH3CO2H,is 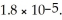
A) 2.42
B) 4.68
C) 9.32
D) 11.58
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 137
Related Exams