B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
200 pm is the same as Å.
A) 2
B) 2000
C) 20
D) 200
E) 2 × 10- 12
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Lithium is a and magnesium is a .
A) metal, metalloid
B) nonmetal, metal
C) nonmetal, nonmetal
D) metalloid, metalloid
E) metal, metal
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Gravitational forces act between objects in proportion to their
A) charges
B) masses
C) volumes
D) densities
E) polarizability
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which pair of substances could be used to illustrate the law of multiple proportions?
A) CO, CO2
B) SO2, H2SO4
C) NaCl, KCl
D) CH4, C6H12O6
E) H2O, O2
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The correct name for SrO is .
A) strontium monoxide
B) strontium dioxide
C) strontium peroxide
D) strontium hydroxide
E) strontium oxide
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Oxygen is a _ and nitrogen is a _.
A) nonmetal, metalloid
B) metal, metalloid
C) metalloid, metalloid
D) nonmetal, nonmetal
E) nonmetal, metal
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The name of the ionic compound NH4CN is _ .
A) cyanonitride
B) nitrogen hydrogen cyanate
C) ammonium cyanide
D) ammonium hydrogen cyanate
E) ammonium carbonitride
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which species has 54 electrons?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is amu. 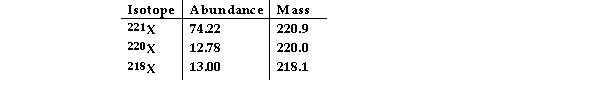
A) 219.7
B) 221.0
C) 220.4
D) 22042
E) 218.5
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Silver has two naturally occurring isotopes with the following isotopic masses:
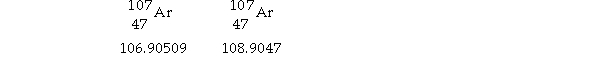 The average atomic mass of silver is 107.8682 amu. The fractional abundance of the lighter of the two isotopes is .
The average atomic mass of silver is 107.8682 amu. The fractional abundance of the lighter of the two isotopes is .
A) 0.7578
B) 0.9047
C) 0.5184
D) 0.2422
E) 0.4816
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atom has the smallest number of neutrons?
A) oxygen- 16
B) neon- 20
C) carbon- 14
D) fluorine- 19
E) nitrogen- 14
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formula of the compound formed between strontium ions and nitrogen ions?
A) Sr2N3
B) SrN3
C) SrN2
D) SrN
E) Sr3N2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mass number of an atom of 118Xe is _ .
A) 64
B) 118
C) 110
D) 172
E) 54
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Potassium is a _ and chlorine is a .
A) nonmetal, metal
B) metal, metalloid
C) metalloid, nonmetal
D) metal, nonmetal
E) metal, metal
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom of 17O contains protons.
A) 17
B) 11
C) 9
D) 25
E) 8
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The name of the ionic compound (NH4) 3PO4 is _ .
A) tetrammonium phosphate
B) ammonia phosphide
C) triammonium phosphate
D) nitrogen hydrogen phosphate
E) ammonium phosphate
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the symbol below, X = 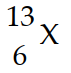
A) C
B) N
C) K
D) Al
E) not enough information to determine
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is not true concerning cathode rays?
A) They impart a negative charge to metals exposed to them.
B) They originate from the negative electrode.
C) They are made up of electrons.
D) The characteristics of cathode rays depend on the material from which they are emitted.
E) They travel in straight lines in the absence of electric or magnetic fields.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The suffix - ide is used .
A) for monatomic anion names
B) for monoatomic cations
C) for polyatomic cation names
D) for the name of the first element in a molecular compound
E) to indicate binary acids
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 201
Related Exams